PDL1 Test for Lung Cancer: Personalized Immunotherapy Solutions
PDL1 Immunotherapy Test
Explore how PDL1 Immunotherapy Test optimizes lung cancer treatment decisions, revolutionizing the way we approach this devastating disease.

Table of Contents
A PDL1 Immunotherapy Test (Programmed Death-Ligand 1), is a type of cancer test that measures the amount of a protein called PD-L1 on cancer cells. PD-L1 is a protein that helps cancer cells evade the immune system. This article provides an overview of the PDL1 test, explains why it is essential, details the testing procedure, outlines necessary preparations, and sheds light on the significance of test results.
Overview
A pd l test measures the presence of a protein called PD-L1 on cancer cells by examining a sample of tumour tissue. It determines the quantity of PD-L1 and its potential impact on cancer treatment known as immuno therapy, which enhances the body’s immune system to combat cancer.
Ordinarily, PD-L1 is present on particular healthy cells and acts as a “brake” to prevent T cell, a component of the immune system, from attacking healthy cells within the body. When cancer cells possess significant levels of PD-L1, they can deactivate T cells, rendering them incapable of attacking the cancerous cells.
If cancer cells exhibit high levels of PD-L1, immune checkpoint inhibitors, a type of immunotherapy medication, may be administered. These drugs hinder the PD-L1 protein’s ability to inhibit T cells, allowing the T cells to effectively fight against cancer.
Immunotherapy can impede or slow down the growth of various PD-L1-positive cancers. Compared to cancer chemotherapy, immunotherapy generally has fewer adverse effects. However, it can result in severe side effects for certain individuals, and not everyone benefits from it.
Multiple PD-L1 tests are available, and the specific test ordered by your healthcare provider depends on the type of cancer you have and the particular medication being considered for your treatment.
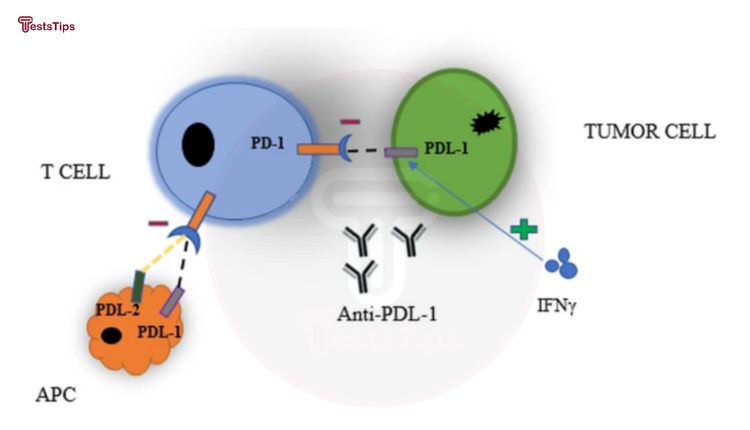
Table of test description
| Test name | Description |
|---|---|
| PDL1 test | Measures the amount of PD-L1 protein on cancer cells |
| Purpose | To determine if you are likely to benefit from immunotherapy |
| Test type | Tissue biopsy |
| Preparation | None required |
| Results | Reported as a percentage |
| Interpretation | A percentage of 50% or higher is considered to be a positive result |
| Other names | PDLI, programmed death-ligand 1, PDL-1 by immunohistochemistry (IHC) |
Why I need the PDL1 Immunotherapy Test?
An anti–PD L1 test may be ordered if you have been diagnosed with cancer:
- Personalized Treatment: The PDL1 (Immunotherapy) Test assists in identifying patients who are most likely to benefit from immunotherapy, allowing for a personalized treatment approach.
- Treatment Decision Making: The test helps oncologists determine whether immuno-therapy should be included as part of a patient’s treatment plan or if alternative therapies may be more suitable.
- Avoiding Unnecessary Side Effects: By identifying patients who are unlikely to respond to immunotherapy, the test helps prevent unnecessary exposure to potential side effects of the treatment.
“Related: Breast Cancer HER2 Testing: What You Need to Know“
What happens during the PDL1 Test?
- Tissue Sample Collection: The test involves obtaining a tissue sample block from the tumor or affected area. This can be done through a biopsy or surgical procedure, depending on the case.
- Lab Analysis: The collected tissue sample is sent to a specialized laboratory where it undergoes detailed assay to determine the level of PDL1 protein expression.
- PDL1 Expression Scoring: The laboratory evaluates the specimens using specific scoring systems, such as the Tumor Proportion Score (TPS) or Combined Positive Score (CPS), to quantify the PDL1 protein expression levels.
- Results Reporting: The oncology laboratory provides a comprehensive report to the pathologists, indicating the PDL1 expression level in the tumor tissue.
Understanding PDL1 Immunotherapy Test Results
The results of a PDL1 test are reported as a percentage. A higher percentage indicates that more cancer cells have PD-L1 on their surface.
- Positive PDL1 Expression: A percentage of 50% or higher is considered to be a positive result, which means that you are more likely to benefit from immunotherapies.
- Negative PDL1 Expression: A low or negative PDL1 expression level suggests that immunotherapy may not provide significant benefits. Alternative treatment options should be explored in such cases.
- Interpretation by Oncologist: The test results are interpreted by the oncologist in conjunction with other clinical factors, such as tumors type, stage, and overall health, to make informed treatment decisions.
Lung cancer
The target of Immunotherapy is the PD-1/PDL1 pathway has revolutionized the treatment landscape for lung cancer, particularly non-small cell lung cancer (NSCLC). Here’s how immunotherapy, including PDL1 testing, is utilized in the management of lung cancer:
Non-Small Cell Lung Cancer (NSCLC):
- PDL1 Testing: PDL1 expression testing is commonly performed in NSCLC patients to determine eligibility for immunotherapy.
- First-line Treatment: In advanced NSCLC cases with high PDL1 expression (usually defined as a TPS cutoff of 50% or higher), immune checkpoint inhibitor targeting PD-1/PDL1 are often used as a first-line treatment option.
- Second-line Treatment: Immunotherapy is also employed as a second-line therapy for NSCLC patients who have progressed after initial chemotherapy or targeted therapy.
- Combination Therapies: Immunotherapy may be combined with chemotherapy, targeted therapy, or other immunotherapeutic agents to enhance treatment efficacy in certain cases.
PDL1 expression testing helps identify patients who are more likely to respond to immunotherapy, allowing for more personalized treatment decisions.
Keytruda
Keytruda, also known by its generic name pembrolizumab, is an FDA-approved immunotherapy drug that has revolutionized the treatment of various types of cancer. It belongs to a class of medications called PD-1 inhibitors, which work by blocking the PD-1 receptor on immune cells, thereby unleashing the body’s immune system to attack cancer cells.
In conclusion, the PDL1 Immunotherapy Test plays a crucial role in guiding personalized cancer treatment decisions. By identifying patients who are likely to respond to immunotherapy, it offers new hope for effective and targeted therapies. Discussing the test with your doctors and understanding the results can empower patients to make informed choices in their cancer treatment journey.
